Boosts Immune Response to Lung Infections and Promotes Easy Breathing
Introduction
Respiratory infection is one of the leading causes of mortality in children under v years of age (1, ii). Early on life respiratory viral infections are virtually usually caused by rhinovirus, respiratory syncytial virus (RSV), influenza, parainfluenza virus, and coronavirus (3). Infection is frequently restricted to the upper respiratory tract but may develop into severe lower respiratory tract infection, such equally RSV bronchiolitis, the leading cause of hospitalization of infants worldwide (4–7). Bacterial pneumonia in infants, acquired by agents such equally Haemophilus influenzae and Streptococcus pneumoniae, is estimated to cause a million deaths in infants under v years of age annually (8, ix). Maternal antibodies afford some protection against infection but wane over the first months of life, and neonates and infants respond poorly to vaccination, leaving early on life as a window of particular vulnerability to respiratory infection (10, 11). Experiences during the crucial neonatal and infant window may shape respiratory health in the long term (12–14). Severe RSV infection in infants is associated with the development of wheeze and asthma in babyhood (xv–xix) and even respiratory affliction that occur late in life, such as chronic obstructive pulmonary disease, are associated with early on life events (twenty–24).
At birth, the neonate emerges from the sheltered intrauterine surround into a plethora of antigenic challenges from pathogens, commensals, and harmless environmental antigens. Neonatal immunity is, in full general, attenuated compared to that of adults (4, 25–29). Differences in amnesty in early life are due to tissue leukopenia, cell intrinsic hyporesponsiveness, and inhibitory mechanisms, such as CD71+ immunosuppressive erythroid cells and high levels of adenosine in extracellular fluids (26, 28–31). Protective Th1 polarized responses and antibodies are produced less well in early life than in adults, along with a propensity to develop unwanted, Th2 or Th17 biased, or dysregulated inflammation (28, 31–33), for instance, following vaccination or allergen exposure (34, 35). TLR stimulation of string blood leukocytes results in a lower production of proinflammatory, Th1-associated cytokines (IL-12p70, TNF-α, IFN-α), and greater product of IL-ten and the Th17-promoting IL-vi and IL-23 when compared to stimulation of developed claret cells, although equivalent responses to TLR vii/8 ligand R848 occur (29, 36, 37). Over the commencement few years of life, antiviral and Th1-biasing cytokine product increases (38, 39).
In the face of an inexperienced adaptive response, innate immunity is probable to play a more than dominant role in protection confronting infection in early life than in machismo. This is supported by the findings that many cistron polymorphisms associated with astringent RSV infection in infants encode components of the innate allowed response (4, 40–43). The importance of TLR signaling in early life is illustrated past individuals with genetic deficiencies in components of the TLR signaling pathway such as MyD88 or IRAK-4. These patients are at high take a chance of bacterial infection in childhood, including in the respiratory tract; however, their condition improves dramatically with historic period (44). This review will focus on describing our current knowledge of innate immunity in the neonatal lung equally a showtime line of defence force against infection. Some potentially important mechanisms underlying susceptibility to lung infection in infants are summarized in Figure 1.
FIGURE i
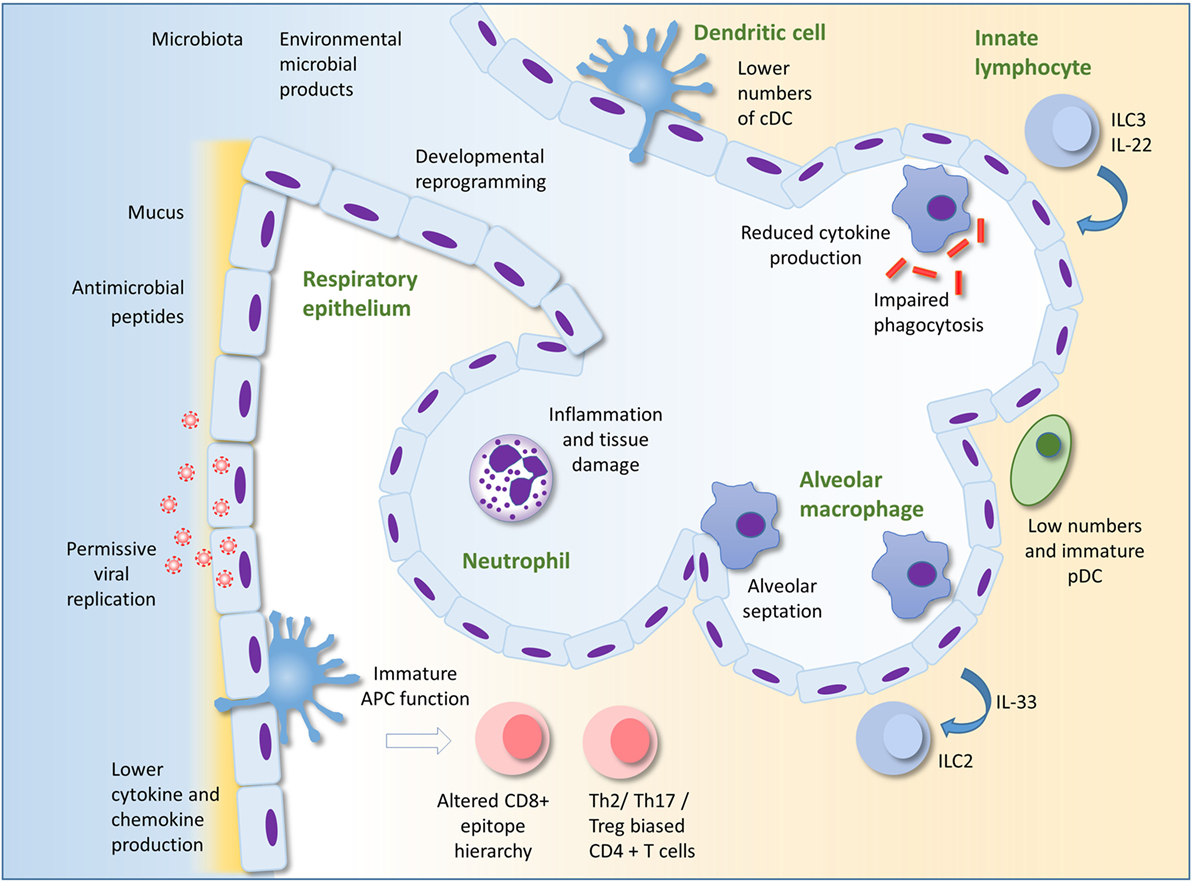
Figure one. Innate immunity to infection in the lung in early life. Alveolar macrophages (AM) are the most numerous leukocyte in the lungs in early life. Reduced cytokine production and phagocytic ability in AM in early life compared to those of adults could underlie susceptibility to infection. AM also promote pre- and post-natal lung development and remodeling. The respiratory epithelium protects against infection through the production of mucus and antimicrobial peptides. Production of type I IFNs may be lower in babe than adult epithelial cells, maybe permitting greater viral replication. Epithelial cells may interact with innate lymphocytes to both initiate and regulate inflammation. Developmental reprograming in the epithelium in early life may also alter the nature of the epithelial response to infection. There are depression numbers of pDC in the lungs compared to adults. Recruitment of neutrophils to the lung occurs less readily in early life compared to adults in some circumstances, but in other situations, excessive recruitment of inflammatory cells can lead to lung inflammation, tissue harm, and impairment of gaseous substitution. Immaturity and lower numbers of dendritic cells, the environment every bit well as intrinsic differences in T cells in early life may result in the development of skewed helper T cell responses and an altered epitope hierarchy in CD8+ T cells. Innate immunity in the lung in early life is influenced by conquering of the microbiota, exposure to microbial products and other environmental factors, as well as the baby genome. Adapted by permission from Macmillan Publishers Ltd: Nature Reviews Immunology (45), copyright 2014.
Respiratory Immunity in Early on Life
It is relatively hard to obtain samples from the lower airways of healthy infant subjects, so many studies have been carried out in murine and other animal models. Information on the cellular limerick of the neonatal lung in humans has come from analysis of bronchoalveolar lavage fluid composition (46–49), immunohistochemistry (50), and more recently, extensive phenotypic analysis of leukocyte subsets in pediatric tissues (51–53).
Adaptive Immunity
Fetal airways are substantially devoid of lymphocytes, they are seeded from nascency, and lymphocytes increase equally a proportion of airway cells over the get-go few years of life (48, 54). There is a relative paucity in CD4+ cells (46, l), and memory T cells are less abundant in infant lungs than in adults, though they are more arable in the lungs than many other tissues (51). Tregs are relatively abundant in pediatric tissues and may have a higher suppressive capacity than those from adults (28, 51) and a transient increment in regulatory T cells, associated with microbial colonization, protects from hyperresponsiveness to allergen (35). A failure of regulation may underlie excessive inflammation in infection, as in RSV bronchiolitis (43), and RSV infection in early life can increase susceptibility to allergic inflammation in the mouse model through an harm of regulatory T cells (4, 55). CD8+ T cells in the lung correlate with disease severity in infants with respiratory failure due to respiratory viral infection (52) and in neonatal mice infected with RSV, a CD8+ T prison cell epitope bureaucracy emerges, which is singled-out to that of adults (56). Singled-out phenotypes of adaptive lymphocytes are plant in early life. A subset of Th cells in human cord claret produce the neutrophil chemoattractant interleukin-viii upon activation (57) and, during RSV infection, a regulatory phenotype in the neonatal B cell compartment may dampen protective immunity (58).
Lung Dendritic Cells (DCs)
At that place is some evidence that neonatal T cells accept the capacity to mount adult-similar protective responses to lung infection. Adoptive transfer of neonatal CD4+ T cells into Pneumocystis carinii-infected adult SCID mice allowed for developed-level pathogen clearance and cytokine product (59, 60), suggesting that the neonatal environment in the lung influences T cell responses. This may exist due in part to the function of neonatal antigen-presenting cells. Neonatal mouse lungs comprise relatively fewer conventional DCs (cDCs), which are immature and poorly functional (56, 61, 62), although mature functions ex vivo accept been reported (63). During neonatal RSV infection, migratory cDCs are dominated by CD103+ DCs, while the CD11b+ contribution increases with age (64). These CD103+ DCs are phenotypically young and poorly functional (65), and this may influence the magnitude and epitope bureaucracy of the CD8+ T cell response (64–66), although these are also influenced by T cell intrinsic differences and regulatory T cells (56, 67). Besides as stimulating protective responses, lung DCs in neonates must promote tolerance to harmless ecology antigens. CD11b+ cDCs in the lung induce Th2 responses to allergens, merely transiently limited loftier levels of PD-L1, which promotes tolerance, following acquisition of the microbiota (35, 68). In contrast to murine studies, the relative frequency of different DC subsets in the human lung appears to exist relatively stable over the life class (53).
In the murine neonatal lung, stiff IFN-α-producing pDC cells are scarce (61), and there is limited recruitment of pDCs and IFN-α production following RSV infection (69).
Alveolar Macrophages (AM)
Lung resident macrophages, which include AM and the less well-characterized interstitial macrophages (70–72), are an of import component of the outset line of defense in the lung. In the steady state, AMs remove debris and maintain a tolerogenic environment; during infection, they secrete proinflammatory cytokines and contribute to pathogen clearance; and after infection, they assistance resolution of inflammation (45). AMs are the predominant cell type in the neonatal airway, they announced in the alveolar compartment from just before nativity and throughout the starting time week of life, and are relatively arable and self-renewing, persisting for at least eleven weeks in mice (47–fifty, 73, 74).
Stimulation of cultured cells has been used to interrogate the relative antimicrobial functions of neonatal and adult AMs. LPS stimulation of rodent or ovine AMs results in like or even enhanced upregulation of TNF-α and CXC-chemokines in neonatal compared to adult cells (75–77), though others demonstrated a reduced translocation of NF-κB to the nucleus of AM from neonatal mice (78). Enhanced phagocytosis by neonatal compared to adult rat AM has been observed (75), but others accept reported impaired phagocytosis and subsequent killing of yeast particles in neonatal rhesus monkey AMs; and dumb phagocytosis of opsonized cherry blood cells in neonatal rat AMs in comparison to adults (79, fourscore). In a murine model of Pneumocystis infection, neonatal AMs were delayed in their expression of activation markers in vivo in comparison to adults (81). Similarly, during murine neonatal RSV infection, there was reduced and delayed AM activation compared to developed infection (82), but intranasal IFN-γ was able to promote AM maturation (82). Little is known about responses in human baby AMs. Cultured cells obtained by bronchoalveolar lavage from infants <2 years of age produce lower IL-1 and TNF-α following LPS stimulation compared with cells from children anile 2–17 (54). The apparent contradictions in the data on AM function in early life may reverberate differences in the species, historic period, experimental weather condition, and assays used. Various macrophage functions are likely to mature at different rates. Neonatal and adult AMs are likely to behave differently in their corresponding lung environments, which is a limitation of these in vitro studies.
Respiratory Epithelial Cells
The respiratory epithelium is the principal site of replication of respiratory viruses. It is in close communication with AM and acts an immune sentinel producing inflammatory mediators, such every bit type I and Iii interferons, mucus, and antimicrobial proteins (45, 83). Relatively little is known about the immunological functions of the airway epithelium in early life. In cultured tracheobronchial epithelial cells from Rhesus macaques of dissimilar ages (infant, juvenile, and adult), IL-8 production on exposure to LPS positively correlated with historic period (84). Furthermore, epithelial cells from juveniles housed in filtered air produced college cytokine responses than those in conventional housing suggesting the microbial richness of the environment may influence epithelial responsiveness. The aforementioned group demonstrated that infant Rhesus monkey primary epithelial cell cultures are more permissive for the H1N1 influenza virus than those from adult airways, while producing less IL-1α (85).
In humans, type I IFNs are detected at merely low levels in the airways of RSV-bronchiolitic infants. This may be due to inhibition of the host anti-viral response past the viral non-structural proteins just alternatively may reflect the timing of sampling, and an IFN-induced gene signature is detectable in claret (86–88). Pediatric nasal and airway epithelial cells cultured from bronchial brushings are readily infected with RSV (89–91) and poor consecration of blazon I IFNs by RSV is reflected in these cultures (92, 93). Instead, the type III interferon IL-29 (IFN-λ) is detected both in the airways of bronchiolitic infants and in cultures of RSV infected airway epithelial cells, and IL-29 pretreatment of cultured epithelial cells attenuates RSV growth (92, 93). Epithelial cells are probably a central source of inflammatory cytokines in respiratory tract secretions of infants with astute RSV (92, 94, 95), including the type-2 immunity promoting cytokine IL-33 (96). The cells used in many in vitro experiments on pediatric respiratory epithelial cells were originally taken from the conducting airway and data surrounding lower airway and ATII cells in early life is even sparser.
Antimicrobial proteins are a outset line of defense force at barrier sites and are produced primarily past epithelial cells and innate leukocytes, particularly neutrophils (97, 98). In the lung, they include surfactants as well as S100s, β-defensins, and cathelicidin and they may provide protection against important infant respiratory infections, including RSV (99–102). Cathelicidin has direct antiviral action against RSV, can forestall infection in vitro and in vivo and in children hospitalized with bronchiolitis, those with low serum cathelicidin were significantly more likely to have RSV infection and a longer hospital stay (97, 103–107).
Innate Lymphocytes
Neonatal murine lungs evidence no quantitative deficiency in γδ T cells as a proportion of CD3+ T cells (61, 108). Exposure to allergen in neonatal mice can stimulate innate ILC2 lymphocytes, a major source of type 2 cytokines (109). Colonization by the microbiota in neonates protects against the accumulation of potentially pro-inflammatory mucosal iNKT cells in the lung and gut (110). Colonization of the gut of neonatal mice can also atomic number 82 to intestinal DC mediated upregulation of CCR4 on IL-22 producing ILC3, which allows their migration into the lungs of neonatal mice, and promotes protection confronting bacterial pneumonia (111).
Neutrophils
Recruitment of innate leukocytes and, in detail, neutrophils, is likely to play an important role in the innate response to infection in the neonatal lung following microbial recognition. Both TLR4 cistron and poly peptide expression are present in the murine lung in the fetus and increase with age through to adulthood (112, 113). TLR2 expression is also present in the human fetal lung and increases with gestational age (114). Information technology appears that there is an immaturity of chemokine production at baseline in the respiratory mucosa. Expression of CXCL2 is low in neonatal mice compared with adults (115) and in uninfected infants (newborn to 18 months), the concentration of IL-eight in nasal washes positively correlates with age (116). There is a dramatically reduced and delayed neutrophil influx in neonatal lung in response to assistants of LPS or leaner in comparison to developed animals (75, 117–119). In the neonatal murine lung, infection with the paramyxovirus Sendai virus results in a minimal early on influx of neutrophils and low product of pro-inflammatory cytokines compared with the adult lung; similarly in murine RSV infection, early pro-inflammatory cytokine production is impaired (108, 115). Macerated recruitment of neutrophils may likewise be due to an impaired chemotaxic power of baby neutrophils (25, 120, 121).
In astringent RSV bronchiolitis in infants, neutrophils tin account for the majority of cells recovered from the airways, associated with increased neutrophil elastase (122–125) and IL-8 (94, 126), although others have reported a lower inflammatory cytokine response in infants with severe vs mild RSV bronchiolitis (127). At that place is a considerable influx of neutrophils into Due south. pneumoniae-infected lungs of neonatal and developed mice, with the neonatal influx even occurring at a lower bacterial dose (128). It is unclear under what circumstances the neonatal lung volition produce an equivalent or exacerbated inflammatory response compared to that of adults, whether this simply requires a loftier level of stimulation or whether boosted factors are involved.
Factors Influencing the Development and Maturation of Lung Amnesty
Despite the credible absence of a mature adult-similar immune system, neonates are able to produce effective allowed responses that defend confronting infection and indeed excessive inflammation tin occur. The neonate must strike a balance betwixt protection against infection and potential impairment to the developing lung and may utilize culling mechanisms of protection against infection to those that predominate in adults.
Exposure to microbial products from the environs, the microbiota, or infection may be beneficial in terms of their ability to promote immune maturation and more than adult like innate and adaptive amnesty (28, thirty). Handling with TLR agonists CpG or LPS during RSV infection alters the CD8+ T cell response toward a more adult-like immunodominance (66) and treatment of neonatal mice with CpG prior to RSV infection shifts the secondary response to re-infection away from a type two response (129). Furthermore, administration of BCG shifts lung CD4+ responses abroad from a Th2 bias and cDC from BCG treated lungs promote Th1 responses (61).
The microbiota is acquired from the mother at nascency and in early life and an developed-like microbiome is established by around 3 years of age (130). The composition of the microbiota and microbial richness of the environment in which children develop have been linked to susceptibility to severe respiratory infections and the evolution of wheeze and asthma (131–133). Ecology microbial exposure may influence lung wellness past establishing the set-signal of immunological responsiveness of the lung, as seen by the attenuation of allergic lung inflammation by airway exposure to LPS or endotoxin rich dust samples (133, 134). Additionally, commensal leaner may influence neonatal respiratory amnesty indirectly. For example, sensing of commensal bacteria by gut DCs promotes resistance to bacterial pneumonia in neonatal mice (111). Factors that shape the microbiota, such as delivery by cesarean section and antibiotic utilise in early life and pregnancy, are likely to profoundly influence the developing immune system (xiv, 135). Other environmental factors that regulate the balance of immunity in the baby respiratory tract may include diet, vitamin D condition, breast feeding, maternal immunity, and exposure to environmental pollutants.
Significant stages of lung evolution occur both before and after nascence and hyporesponsiveness to immune stimuli may have evolved to protect the developing lung from the disruptive and damaging effects of inflammation (136, 137). This is evidenced in mouse models of chorioamnionitis, where exposure of the fetal lung to LPS results in abnormal development of the distal airways (138, 139). In addition, IL-1β expression in the fetal or newborn lung impairs normal postnatal development (140). Reciprocally, the developmental programmes active in resident lung cells, which drive cell growth and differentiation may also influence immune responses (141, 142). Macrophages take on important roles in lung evolution and remodeling including septation and vascularization of the alveoli after nascency (137, 143). Macrophages acquaintance with sites of branching morphogenesis where they assume a tissue remodeling phenotype and promote development through production of growth factors and matrix metalloproteases (143). Polarization of macrophages abroad from this phenotype might, therefore, be a mechanism past which pro-inflammatory signals disrupt lung development (138, 140). As with lung macrophages, the respiratory epithelium will be subject to lung developmental programmes extending into the postnatal period, which regulate epithelial prison cell proliferation and differentiation, and these may potentially also modify epithelial immunological function. Foxa2 is an epithelially expressed member of the forkhead family of transcription factors. In the developing lung, it regulates epithelial differentiation and controls goblet cell hyperplasia. It likewise has immunoregulatory functions and limits type-two immunity through inhibition of the cysteinyl LT signaling pathway (83, 141, 144).
Decision
The mechanisms that regulate inflammatory responses to microbial stimulation in the lung demand to be more fully elucidated. Increasing our knowledge of how the developing immune organisation responds to infectious challenge is of importance for development of neonatal vaccines and treatments for exaggerated respiratory inflammation during infection. In certain circumstances, the allowed organisation in early on life is capable of developed-level responses, and maybe boosting responses in at-risk infants—in treatment for acute communicable diseases or equally adjuvant for vaccination—would be a beneficial protective strategy. Additionally, selectively harnessing the protective innate mechanisms that are already expressed at adult or greater than developed levels in the neonate could exist a safe therapeutic method. Thus, while early life is clearly a period of immunological vulnerability for the developing lung, it is likewise an opportunity for effective intervention strategies, which could benefit respiratory health not only in infancy, but into machismo.
Writer Contributions
LL researched the literature and wrote the review. FC wrote the review, edited, and updated it.
Conflict of Interest Statement
The authors declare that the enquiry was conducted in the absence of whatsoever commercial or fiscal relationships that could be construed as a potential conflict of involvement.
Acknowledgments
Some of the content of this manuscript first appeared in Lambert (2015) (145), Immunology of the Neonatal Lung and the Long Term Consequences of Neonatal Respiratory Virus Infection for Pulmonary Innate Immunity. Ph.D. thesis: Imperial College London. We thank Dr. Spiros Makris for critical reading of the manuscript. This piece of work was supported past a Medical Research Council New Investigator Research Grant to FC (G1001763).
References
i. Liu L, Oza South, Hogan D, Perin J, Rudan I, Backyard JE, et al. Global, regional, and national causes of kid mortality in 2000-13, with projections to inform mail-2015 priorities: an updated systematic assay. Lancet (2015) 385(9966):430–twoscore. doi:ten.1016/S0140-6736(14)61698-half-dozen
PubMed Abstruse | CrossRef Total Text | Google Scholar
two. Walker CLF, Rudan I, Liu L, Nair H, Theodoratou Eastward, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet (2013) 381(9875):1405–16. doi:10.1016/S0140-6736(thirteen)60222-6
CrossRef Full Text | Google Scholar
iii. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev (2010) 23(one):74–98. doi:10.1128/CMR.00032-09
CrossRef Full Text | Google Scholar
five. Nair H, Nokes DJ, Gessner BD, Dherani Thou, Madhi SA, Singleton RJ, et al. Global brunt of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet (2010) 375(9725):1545–55. doi:ten.1016/S0140-6736(10)60206-i
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. Due north Engl J Med (2009) 360(6):588–98. doi:x.1056/NEJMoa0804877
PubMed Abstruse | CrossRef Full Text | Google Scholar
eight. O'Brien KL, Wolfson LJ, Watt JP, Henkle Eastward, Deloria-Knoll 1000, McCall Northward, et al. Brunt of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet (2009) 374(9693):893–902. doi:10.1016/S0140-6736(09)61204-6
PubMed Abstract | CrossRef Full Text | Google Scholar
nine. Watt JP, Wolfson LJ, O'Brien KL, Henkle E, Deloria-Knoll Thousand, McCall North, et al. Burden of illness caused past Haemophilus influenzae type b in children younger than five years: global estimates. Lancet (2009) 374(9693):903–11. doi:10.1016/S0140-6736(09)61203-4
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Heath PT, Culley FJ, Jones CE, Kampmann B, Le Doare 1000, Nunes MC, et al. Grouping B streptococcus and respiratory syncytial virus immunisation during pregnancy: a landscape analysis. Lancet Infect Dis (2017) 17(7):e223–34. doi:x.1016/S1473-3099(17)30232-3
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Renz H, Brandtzaeg P, Hornef M. The bear on of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol (2011) 12(1):9–23. doi:x.1038/nri3112
PubMed Abstract | CrossRef Total Text | Google Scholar
xiv. Man WH, de Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol (2017) fifteen(five):259–70. doi:10.1038/nrmicro.2017.fourteen
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Biesbroek Thousand, Tsivtsivadze Due east, Sanders EAM, Montijn R, Veenhoven RH, Keijser BJF, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory wellness in children. Am J Respir Crit Care Med (2014) 190(11):1283–92. doi:10.1164/rccm.201407-1240OC
PubMed Abstract | CrossRef Full Text | Google Scholar
xvi. De Steenhuijsen Piters WAA, Heinonen Southward, Hasrat R, Bunsow Due east, Smith B, Suarez-Arrabal M-C, et al. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Intendance Med (2016) 194(9):1104–15. doi:10.1164/rccm.201602-0220OC
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med (2005) 171(ii):137–41. doi:x.1164/rccm.200406-730OC
PubMed Abstruse | CrossRef Full Text | Google Scholar
18. Sigurs Northward, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the offset year of life. Thorax (2010) 65(12):1045–52. doi:10.1136/thx.2009.121582
PubMed Abstract | CrossRef Total Text | Google Scholar
19. Caballero MT, Serra ME, Acosta PL, Marzec J, Gibbons L, Salim G, et al. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest (2015) 125(2):571–82. doi:10.1172/JCI75183
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Svanes C, Sunyer J, Plana East, Dharmage South, Heinrich J, Jarvis D, et al. Early on life origins of chronic obstructive pulmonary disease. Thorax (2010) 65(i):14–20. doi:10.1136/thx.2008.112136
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Shaheen SO, Barker DJ, Holgate ST. Do lower respiratory tract infections in early childhood cause chronic obstructive pulmonary illness? Am J Respir Crit Care Med (1995) 151(five):1649–51. doi:10.1164/ajrccm/151.5_Pt_1.1649
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Bui DS, Burgess JA, Lowe AJ, Perret JL, Lodge CJ, Bui Yard, et al. Babyhood lung function predicts adult chronic obstructive pulmonary disease and asthma-chronic obstructive pulmonary disease overlap syndrome. Am J Respir Crit Care Med (2017) 196(1):39–46. doi:ten.1164/rccm.201606-1272OC
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and immature babe from infectious diseases: lessons from immune ontogeny. Immunity (2017) 46(3):350–63. doi:x.1016/j.immuni.2017.03.009
PubMed Abstruse | CrossRef Full Text | Google Scholar
29. Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune office by Price-like receptors: distinct responses in newborns and the elderly. Immunity (2012) 37(v):771–83. doi:10.1016/j.immuni.2012.ten.014
PubMed Abstruse | CrossRef Full Text | Google Scholar
31. Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol (2004) 4(seven):553–64. doi:10.1038/nri1394
CrossRef Total Text | Google Scholar
33. PrabhuDas G, Adkins B, Gans H, Rex C, Levy O, Ramilo O, et al. Challenges in baby immunity: implications for responses to infection and vaccines. Nat Immunol (2011) 12(3):189–94. doi:10.1038/ni0311-189
CrossRef Full Text | Google Scholar
34. Saglani South, Mathie SA, Gregory LG, Bell MJ, Bush-league A, Lloyd CM. Pathophysiological features of asthma develop in parallel in house dust mite-exposed neonatal mice. Am J Respir Cell Mol Biol (2009) 41(3):281–9. doi:10.1165/rcmb.2008-0396OC
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Gollwitzer ES, Saglani Southward, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med (2014) 20(6):642–7. doi:10.1038/nm.3568
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate amnesty in human being newborns: neonatal claret plasma reduces monocyte TNF-blastoff induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol (2004) 173(vii):4627–34. doi:ten.4049/jimmunol.173.7.4627
PubMed Abstruse | CrossRef Full Text | Google Scholar
37. Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol (2009) 183(11):7150–60. doi:10.4049/jimmunol.0901481
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, et al. Ontogeny of Toll-similar receptor mediated cytokine responses of human being blood mononuclear cells. PLoS One (2010) v(11):e15041. doi:10.1371/journal.pone.0015041
PubMed Abstract | CrossRef Total Text | Google Scholar
39. Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, et al. Skewed blueprint of Cost-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and loftier IL-ten persist throughout the first calendar month of life. Clin Immunol (2009) 133(2):228–37. doi:10.1016/j.clim.2009.07.003
PubMed Abstract | CrossRef Full Text | Google Scholar
xl. Faber TE, Schuurhof A, Vonk A, Koppelman GH, Hennus MP, Kimpen JLL, et al. IL1RL1 gene variants and nasopharyngeal IL1RL-a levels are associated with severe RSV bronchiolitis: a multicenter cohort report. PLoS One (2012) 7(5):e34364. doi:10.1371/journal.pone.0034364
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Janssen R, Bont L, Siezen CLE, Hodemaekers HM, Ermers MJ, Doornbos Thou, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis (2007) 196(vi):826–34. doi:x.1086/520886
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Tulic MK, Hurrelbrink RJ, Prêle CM, Laing IA, Upham JW, Le Souef P, et al. TLR4 polymorphisms mediate dumb responses to respiratory syncytial virus and lipopolysaccharide. J Immunol (2007) 179(one):132–40. doi:10.4049/jimmunol.179.ane.132
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Ratjen F, Bredendiek 1000, Zheng 50, Brendel M, Costabel U. Lymphocyte subsets in bronchoalveolar lavage fluid of children without bronchopulmonary illness. Am J Respir Crit Care Med (1995) 152(one):174–8. doi:10.1164/ajrccm.152.i.7599820
PubMed Abstract | CrossRef Full Text | Google Scholar
48. Grigg J, Riedler J. Developmental airway cell biology. The "normal" young child. Am J Respir Crit Care Med (2000) 162(two Pt 2):S52–5. doi:10.1164/ajrccm.162.supplement_1.maic-fourteen
CrossRef Full Text | Google Scholar
49. Ratjen F, Bredendiek One thousand, Brendel Chiliad, Meltzer J, Costabel U. Differential cytology of bronchoalveolar lavage fluid in normal children. Eur Respir J (1994) 7(ten):1865–70. doi:10.1183/09031936.94.07101865
PubMed Abstract | CrossRef Full Text | Google Scholar
l. Dos Santos ABG, Binoki D, Silva LFF, de Araujo BB, Otter ID, Annoni R, et al. Immune cell contour in infants' lung tissue. Ann Anat (2013) 195(6):596–604. doi:10.1016/j.aanat.2013.05.003
CrossRef Full Text | Google Scholar
51. Thome JJC, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, et al. Early-life compartmentalization of human T cell differentiation and regulatory role in mucosal and lymphoid tissues. Nat Med (2016) 22(ane):72–7. doi:x.1038/nm.4008
PubMed Abstruse | CrossRef Full Text | Google Scholar
52. Connors TJ, Ravindranath TM, Bickham KL, Gordon CL, Zhang F, Levin B, et al. Airway CD8(+) T cells are associated with lung injury during infant viral respiratory tract infection. Am J Respir Cell Mol Biol (2016) 54(half dozen):822–xxx. doi:10.1165/rcmb.2015-0297OC
PubMed Abstract | CrossRef Full Text | Google Scholar
53. Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, et al. Dendritic cells display subset and tissue-specific maturation dynamics over homo life. Immunity (2017) 46(3):504–15. doi:10.1016/j.immuni.2017.02.019
PubMed Abstract | CrossRef Full Text | Google Scholar
54. Grigg J, Riedler J, Robertson CF, Boyle W, Uren S. Alveolar macrophage immaturity in infants and immature children. Eur Respir J (1999) 14(five):1198–205. doi:x.1183/09031936.99.14511989
PubMed Abstract | CrossRef Full Text | Google Scholar
55. Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med (2012) 18(ten):1525–30. doi:10.1038/nm.2896
PubMed Abstract | CrossRef Full Text | Google Scholar
56. Ruckwardt TJ, Malloy AMW, Gostick Due east, Price DA, Dash P, McClaren JL, et al. Neonatal CD8 T-cell bureaucracy is distinct from adults and is influenced by intrinsic T prison cell properties in respiratory syncytial virus infected mice. PLoS Pathog (2011) 7(12):e1002377. doi:10.1371/journal.ppat.1002377
PubMed Abstract | CrossRef Full Text | Google Scholar
57. Gibbons D, Fleming P, Virasami A, Michel M-50, Sebire NJ, Costeloe K, et al. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med (2014) 20(ten):1206–10. doi:ten.1038/nm.3670
PubMed Abstract | CrossRef Full Text | Google Scholar
58. Zhivaki D, Lemoine S, Lim A, Morva A, Vidalain P-O, Schandene L, et al. Respiratory syncytial virus infects regulatory B cells in homo neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity (2017) 46(2):301–14. doi:x.1016/j.immuni.2017.01.010
PubMed Abstract | CrossRef Full Text | Google Scholar
59. Qureshi MH, Garvy BA. Neonatal T cells in an developed lung environment are competent to resolve Pneumocystis carinii pneumonia. J Immunol (2001) 166(ix):5704–eleven. doi:ten.4049/jimmunol.166.nine.5704
PubMed Abstract | CrossRef Full Text | Google Scholar
60. Garvy BA, Qureshi MH. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J Immunol (2000) 165(xi):6480–half-dozen. doi:10.4049/jimmunol.165.11.6480
PubMed Abstract | CrossRef Full Text | Google Scholar
61. Roux X, Remot A, Petit-Camurdan A, Nahori Thou-A, Kiefer-Biasizzo H, Marchal M, et al. Neonatal lung immune responses show a shift of cytokines and transcription factors toward Th2 and a deficit in conventional and plasmacytoid dendritic cells. Eur J Immunol (2011) 41(ten):2852–61. doi:ten.1002/eji.201041224
PubMed Abstract | CrossRef Full Text | Google Scholar
62. Nelson DJ, Holt PG. Defective regional amnesty in the respiratory tract of neonates is attributable to hyporesponsiveness of local dendritic cells to activation signals. J Immunol (1995) 155(7):3517–24.
PubMed Abstract | Google Scholar
63. Fach SJ, Brockmeier SL, Hobbs LA, Lehmkuhl HD, Sacco RE. Pulmonary dendritic cells isolated from neonatal and adult ovine lung tissue. Vet Immunol Immunopathol (2006) 112(iii–4):171–82. doi:10.1016/j.vetimm.2006.02.012
PubMed Abstract | CrossRef Total Text | Google Scholar
64. Ruckwardt TJ, Malloy AMW, Morabito KM, Graham BS. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog (2014) 10(ii):e1003934. doi:10.1371/journal.ppat.1003934
PubMed Abstract | CrossRef Full Text | Google Scholar
65. Ruckwardt TJ, Morabito KM, Bar-Haim Due east, Nair D, Graham BS. Neonatal mice possess 2 phenotypically and functionally distinct lung-migratory CD103(+) dendritic prison cell populations following respiratory infection. Mucosal Immunol (2017). doi:x.1038/mi.2017.28
CrossRef Full Text | Google Scholar
66. Malloy AMW, Ruckwardt TJ, Morabito KM, Lau-Kilby AW, Graham BS. Pulmonary dendritic cell subsets shape the respiratory syncytial virus-specific CD8+ T cell immunodominance hierarchy in neonates. J Immunol (2017) 198(1):394–403. doi:10.4049/jimmunol.1600486
PubMed Abstruse | CrossRef Full Text | Google Scholar
67. Ruckwardt TJ, Bonaparte KL, Nason MC, Graham BS. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J Virol (2009) 83(seven):3019–28. doi:10.1128/JVI.00036-09
PubMed Abstract | CrossRef Total Text | Google Scholar
68. Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med (2011) 184(2):198–205. doi:10.1164/rccm.201010-1574OC
PubMed Abstract | CrossRef Full Text | Google Scholar
69. Cormier SA, Shrestha B, Saravia J, Lee GI, Shen 50, DeVincenzo JP, et al. Limited blazon I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection allow immunopathogenesis upon reinfection. J Virol (2014) 88(sixteen):9350–60. doi:ten.1128/JVI.00818-14
PubMed Abstruse | CrossRef Full Text | Google Scholar
70. Guilliams Grand, Lambrecht BN, Hammad H. Partitioning of labor betwixt lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol (2013) half dozen(three):464–73. doi:ten.1038/mi.2013.xiv
PubMed Abstract | CrossRef Full Text | Google Scholar
71. Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry Due east, et al. Lung interstitial macrophages change dendritic cell functions to prevent airway allergy in mice. J Clin Invest (2009) 119(12):3723–38. doi:10.1172/JCI39717
PubMed Abstract | CrossRef Full Text | Google Scholar
72. Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov Due south, Fernandes C, et al. Exposure to bacterial CpG Dna protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity (2017) 46(3):457–73. doi:10.1016/j.immuni.2017.02.016
PubMed Abstruse | CrossRef Full Text | Google Scholar
74. Guilliams M, De Kleer I, Henri Southward, Post Due south, Vanhoutte L, De Prijck Due south, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med (2013) 210(10):1977–92. doi:10.1084/jem.20131199
PubMed Abstract | CrossRef Full Text | Google Scholar
75. Lee PT, Holt PG, McWilliam AS. Role of alveolar macrophages in innate amnesty in neonates: evidence for selective lipopolysaccharide binding protein product by rat neonatal alveolar macrophages. Am J Respir Cell Mol Biol (2000) 23(five):652–61. doi:x.1165/ajrcmb.23.five.4016
PubMed Abstract | CrossRef Full Text | Google Scholar
76. Empey KM, Hollifield M, Garvy BA. Exogenous heat-killed Escherichia coli improves alveolar macrophage activity and reduces Pneumocystis carinii lung burden in infant mice. Infect Immun (2007) 75(7):3382–93. doi:10.1128/IAI.00174-07
PubMed Abstract | CrossRef Full Text | Google Scholar
77. Fach SJ, Olivier A, Gallup JM, Waters TE, Ackermann MR, Lehmkuhl HD, et al. Differential expression of cytokine transcripts in neonatal and adult ovine alveolar macrophages in response to respiratory syncytial virus or price-like receptor ligation. Vet Immunol Immunopathol (2010) 136(1–two):55–64. doi:10.1016/j.vetimm.2010.02.008
PubMed Abstract | CrossRef Full Text | Google Scholar
78. Kurkjian C, Hollifield M, Lines JL, Rogosky A, Empey KM, Qureshi Chiliad, et al. Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection. Infect Immun (2012) 80(8):2835–46. doi:10.1128/IAI.05707-xi
PubMed Abstract | CrossRef Total Text | Google Scholar
79. Ballinger MN, Peters-Golden M, Moore BB. Impaired neonatal macrophage phagocytosis is not explained by overproduction of prostaglandin E2. Respir Res (2011) 12:155. doi:x.1186/1465-9921-12-155
PubMed Abstruse | CrossRef Total Text | Google Scholar
eighty. Kurland One thousand, Cheung AT, Miller ME, Ayin SA, Cho MM, Ford EW. The ontogeny of pulmonary defenses: alveolar macrophage function in neonatal and juvenile rhesus monkeys. Pediatr Res (1988) 23(3):293–7. doi:ten.1203/00006450-198803000-00013
PubMed Abstruse | CrossRef Full Text | Google Scholar
81. Empey KM, Hollifield Thousand, Schuer K, Gigliotti F, Garvy BA. Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation. Infect Immun (2004) 72(11):6211–20. doi:10.1128/IAI.72.eleven.6211-6220.2004
PubMed Abstract | CrossRef Total Text | Google Scholar
82. Empey KM, Orend JG, Peebles RS, Egaña L, Norris KA, Oury TD, et al. Stimulation of immature lung macrophages with intranasal interferon gamma in a novel neonatal mouse model of respiratory syncytial virus infection. PLoS One (2012) 7(7):e40499. doi:x.1371/journal.pone.0040499
PubMed Abstract | CrossRef Total Text | Google Scholar
84. Maniar-Hew Thousand, Clay CC, Postlethwait EM, Evans MJ, Fontaine JH, Miller LA. Innate immune response to LPS in airway epithelium is dependent on chronological age and antecedent exposures. Am J Respir Cell Mol Biol (2013) 49(5):710–xx. doi:x.1165/rcmb.2012-0321OC
PubMed Abstruse | CrossRef Full Text | Google Scholar
85. Dirt CC, Reader JR, Gerriets JE, Wang TT, Harrod KS, Miller LA. Enhanced viral replication and modulated innate immune responses in baby airway epithelium following H1N1 infection. J Virol (2014) 88(13):7412–25. doi:10.1128/JVI.00188-14
PubMed Abstract | CrossRef Total Text | Google Scholar
88. Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, et al. Whole blood gene expression profiles to assess pathogenesis and affliction severity in infants with respiratory syncytial virus infection. PLoS Med (2013) 10(11):e1001549. doi:x.1371/periodical.pmed.1001549
PubMed Abstruse | CrossRef Total Text | Google Scholar
89. Villenave R, Thavagnanam S, Sarlang S, Parker J, Douglas I, Skibinski G, et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc Natl Acad Sci U S A (2012) 109(xiii):5040–5. doi:ten.1073/pnas.1110203109
PubMed Abstract | CrossRef Full Text | Google Scholar
90. Fonceca AM, Flanagan BF, Trinick R, Smyth RL, McNamara PS. Primary airway epithelial cultures from children are highly permissive to respiratory syncytial virus infection. Thorax (2012) 67(ane):42–8. doi:ten.1136/thoraxjnl-2011-200131
PubMed Abstruse | CrossRef Full Text | Google Scholar
92. Villenave R, Broadbent 50, Douglas I, Lyons JD, Coyle PV, Teng MN, et al. Induction and antagonism of antiviral responses in respiratory syncytial virus-infected pediatric airway epithelium. J Virol (2015) 89(24):12309–18. doi:10.1128/JVI.02119-xv
PubMed Abstract | CrossRef Full Text | Google Scholar
93. Okabayashi T, Kojima T, Masaki T, Yokota Due south-I, Imaizumi T, Tsutsumi H, et al. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res (2011) 160(i–2):360–6. doi:x.1016/j.virusres.2011.07.011
PubMed Abstract | CrossRef Total Text | Google Scholar
94. McNamara PS, Flanagan BF, Hart CA, Smyth RL. Product of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis (2005) 191(8):1225–32. doi:10.1086/428855
CrossRef Full Text | Google Scholar
95. McNamara PS, Fonceca AM, Howarth D, Correia JB, Slupsky JR, Trinick RE, et al. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax (2013) 68(1):76–81. doi:10.1136/thoraxjnl-2012-202288
CrossRef Total Text | Google Scholar
96. Saravia J, You lot D, Shrestha B, Jaligama S, Siefker D, Lee GI, et al. Respiratory syncytial virus disease is mediated by age-variable IL-33. PLoS Pathog (2015) 11(10):e1005217. doi:x.1371/journal.ppat.1005217
PubMed Abstruse | CrossRef Total Text | Google Scholar
97. Hasegawa Chiliad, Mansbach JM, Ajami NJ, Petrosino JF, Freishtat RJ, Teach SJ, et al. Serum cathelicidin, nasopharyngeal microbiota, and disease severity among infants hospitalized with bronchiolitis. J Allergy Clin Immunol (2017) 139(4):1383.e–6.e. doi:10.1016/j.jaci.2016.09.037
CrossRef Full Text | Google Scholar
98. Starner TD, Agerberth B, Gudmundsson GH, McCray PB. Expression and activity of beta-defensins and LL-37 in the developing human lung. J Immunol (2005) 174(iii):1608–15. doi:10.4049/jimmunol.174.3.1608
PubMed Abstract | CrossRef Full Text | Google Scholar
99. Schaller-Bals Due south, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med (2002) 165(seven):992–5. doi:ten.1164/ajrccm.165.7.200110-020
PubMed Abstract | CrossRef Total Text | Google Scholar
100. LeVine AM, Gwozdz J, Stark J, Bruno M, Whitsett J, Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J Clin Invest (1999) 103(7):1015–21. doi:10.1172/JCI5849
PubMed Abstract | CrossRef Total Text | Google Scholar
101. Derscheid RJ, Ackermann MR. The innate immune organisation of the perinatal lung and responses to respiratory syncytial virus infection. Vet Pathol (2013) 50(5):827–41. doi:10.1177/0300985813480216
PubMed Abstract | CrossRef Full Text | Google Scholar
102. Battersby AJ, Khara J, Wright VJ, Levy O, Kampmann B. Antimicrobial proteins and peptides in early on life: ontogeny and translational opportunities. Forepart Immunol (2016) 7:309. doi:ten.3389/fimmu.2016.00309
PubMed Abstract | CrossRef Full Text | Google Scholar
103. Mansbach JM, Piedra PA, Stevenson MD, Sullivan AF, Forgey TF, Clark Southward, et al. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics (2012) 130(3):e492–500. doi:10.1542/peds.2012-0444
PubMed Abstract | CrossRef Total Text | Google Scholar
104. Mansbach JM, Piedra PA, Borregaard N, Martineau AR, Neuman MI, Espinola JA, et al. Serum cathelicidin level is associated with viral etiology and severity of bronchiolitis. J Allergy Clin Immunol (2012) 130(iv):1007.e–8.e. doi:10.1016/j.jaci.2012.07.044
CrossRef Full Text | Google Scholar
105. Currie SM, Gwyer Findlay E, McFarlane AJ, Fitch PM, Böttcher B, Colegrave N, et al. Cathelicidins have direct antiviral action against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J Immunol (2016) 196(6):2699–710. doi:10.4049/jimmunol.1502478
PubMed Abstract | CrossRef Full Text | Google Scholar
106. Currie SM, Findlay EG, McHugh BJ, Mackellar A, Man T, Macmillan D, et al. The human cathelicidin LL-37 has antiviral activity confronting respiratory syncytial virus. PLoS One (2013) 8(8):e73659. doi:10.1371/periodical.pone.0073659
PubMed Abstract | CrossRef Full Text | Google Scholar
107. Harcourt JL, McDonald M, Svoboda P, Pohl J, Tatti K, Haynes LM. Human being cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC Res Notes (2016) 9(one):eleven. doi:10.1186/s13104-015-1836-y
PubMed Abstract | CrossRef Full Text | Google Scholar
108. Huang H, Saravia J, You D, Shaw AJ, Cormier SA. Impaired gamma delta T cell-derived IL-17A and inflammasome activation during early respiratory syncytial virus infection in infants. Immunol Jail cell Biol (2015) 93(2):126–35. doi:10.1038/icb.2014.79
PubMed Abstract | CrossRef Full Text | Google Scholar
109. Castanhinha South, Sherburn R, Walker S, Gupta A, Bossley CJ, Buckley J, et al. Pediatric astringent asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol (2015) 136(ii):312.e–22.e. doi:10.1016/j.jaci.2015.01.016
PubMed Abstract | CrossRef Full Text | Google Scholar
110. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T jail cell function. Science (2012) 336(6080):489–93. doi:10.1126/science.1219328
PubMed Abstract | CrossRef Full Text | Google Scholar
111. Grey J, Oehrle K, Worthen G, Alenghat T, Whitsett J, Deshmukh H. Abdominal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci Transl Med (2017) 9(376):eaaf9412. doi:10.1126/scitranslmed.aaf9412
PubMed Abstract | CrossRef Total Text | Google Scholar
113. Harju K, Ojaniemi M, Rounioja S, Glumoff V, Paananen R, Vuolteenaho R, et al. Expression of cost-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr Res (2005) 57(5 Pt 1):644–8. doi:ten.1203/01.PDR.0000156212.03459.A9
PubMed Abstract | CrossRef Total Text | Google Scholar
114. Petrikin JE, Gaedigk R, Leeder JS, Truog We. Selective Cost-similar receptor expression in human fetal lung. Pediatr Res (2010) 68(iv):335–8. doi:10.1203/PDR.0b013e3181ed1134
CrossRef Full Text | Google Scholar
115. Bhattacharya South, Beal BT, Janowski AM, Shornick LP. Reduced inflammation and contradistinct innate response in neonates during paramyxoviral infection. Virol J (2011) viii:549. doi:10.1186/1743-422X-viii-549
PubMed Abstruse | CrossRef Total Text | Google Scholar
116. Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, et al. Relationships among specific viral pathogens, virus-induced interleukin-eight, and respiratory symptoms in infancy. Pediatr Allergy Immunol (2002) thirteen(6):386–93. doi:ten.1034/j.1399-3038.2002.01093.x
PubMed Abstract | CrossRef Full Text | Google Scholar
117. Martin TR, Ruzinski JT, Wilson CB, Skerrett SJ. Effects of endotoxin in the lungs of neonatal rats: age-dependent damage of the inflammatory response. J Infect Dis (1995) 171(1):134–44. doi:ten.1093/infdis/171.1.134
PubMed Abstract | CrossRef Total Text | Google Scholar
118. Martin TR, Rubens CE, Wilson CB. Lung antibacterial defense mechanisms in infant and adult rats: implications for the pathogenesis of group B streptococcal infections in the neonatal lung. J Infect Dis (1988) 157(1):91–100. doi:10.1093/infdis/157.1.91
PubMed Abstract | CrossRef Full Text | Google Scholar
119. McGrath-Morrow SA, Lee S, Gibbs K, Lopez A, Collaco JM, Neptune E, et al. Immune response to intrapharyngeal LPS in neonatal and juvenile mice. Am J Respir Cell Mol Biol (2015) 52(3):323–31. doi:x.1165/rcmb.2014-0100OC
PubMed Abstract | CrossRef Full Text | Google Scholar
120. Anderson DC, Rothlein R, Marlin SD, Krater SS, Smith CW. Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Claret (1990) 76(12):2613–21.
PubMed Abstract | Google Scholar
122. Smith PK, Wang SZ, Dowling KD, Forsyth KD. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatr Child Health (2001) 37(2):146–51. doi:10.1046/j.1440-1754.2001.00618.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
123. McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Curvation Dis Child (2003) 88(10):922–six. doi:10.1136/adc.88.ten.922
PubMed Abstract | CrossRef Total Text | Google Scholar
124. Abu-Harb M, Bell F, Finn A, Rao WH, Nixon Fifty, Shale D, et al. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J (1999) 14(1):139–43. doi:10.1034/j.1399-3003.1999.14a23.x
PubMed Abstract | CrossRef Full Text | Google Scholar
125. Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, et al. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Kid (1994) 71(5):428–32. doi:x.1136/adc.71.5.428
PubMed Abstract | CrossRef Full Text | Google Scholar
126. Noah TL, Ivins SS, Murphy P, Kazachkova I, Moats-Staats B, Henderson FW. Chemokines and inflammation in the nasal passages of infants with respiratory syncytial virus bronchiolitis. Clin Immunol (2002) 104(one):86–95. doi:10.1006/clim.2002.5248
PubMed Abstract | CrossRef Full Text | Google Scholar
127. Nicholson EG, Schlegel C, Garofalo RP, Mehta R, Scheffler 1000, Mei M, et al. Robust cytokine and chemokine response in nasopharyngeal secretions: association with decreased severity in children with doctor diagnosed bronchiolitis. J Infect Dis (2016) 214(4):649–55. doi:10.1093/infdis/jiw191
PubMed Abstract | CrossRef Full Text | Google Scholar
128. Garvy BA, Harmsen AG. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of allowed or naive adults. Infect Immun (1996) 64(10):3987–92.
PubMed Abstract | Google Scholar
129. Yamaguchi Y, Harker JA, Wang B, Openshaw PJ, Tregoning JS, Culley FJ. Preexposure to CpG protects against the delayed furnishings of neonatal respiratory syncytial virus infection. J Virol (2012) 86(19):10456–61. doi:10.1128/JVI.01082-12
PubMed Abstract | CrossRef Total Text | Google Scholar
130. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Man gut microbiome viewed across age and geography. Nature (2012) 486(7402):222–7. doi:ten.1038/nature11053
PubMed Abstract | CrossRef Full Text | Google Scholar
131. Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger Eastward, et al. Prenatal subcontract exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol (2006) 117(4):817–23. doi:ten.1016/j.jaci.2005.12.1307
PubMed Abstract | CrossRef Full Text | Google Scholar
132. Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize Fifty, et al. Ecology exposure to endotoxin and its relation to asthma in school-historic period children. N Engl J Med (2002) 347(12):869–77. doi:10.1056/NEJMoa020057
PubMed Abstract | CrossRef Full Text | Google Scholar
133. Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk 5, Murray SE, et al. Innate immunity and asthma gamble in amish and hutterite farm children. North Engl J Med (2016) 375(v):411–21. doi:10.1056/NEJMoa1508749
PubMed Abstruse | CrossRef Full Text | Google Scholar
134. Schuijs MJ, Willart MA, Vergote M, Gras D, Deswarte Thousand, Ege MJ, et al. Farm grit and endotoxin protect confronting allergy through A20 induction in lung epithelial cells. Scientific discipline (2015) 349(6252):1106–ten. doi:10.1126/science.aac6623
PubMed Abstract | CrossRef Full Text | Google Scholar
135. Schulfer A, Blaser MJ. Risks of antibiotic exposures early on in life on the developing microbiome. PLoS Pathog (2015) 11(7):e1004903. doi:10.1371/journal.ppat.1004903
CrossRef Full Text | Google Scholar
138. Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, et al. NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol (2011) 187(v):2740–vii. doi:10.4049/jimmunol.1101495
PubMed Abstract | CrossRef Full Text | Google Scholar
139. Prince LS, Dieperink Hullo, Okoh VO, Fierro-Perez GA, Lallone RL. Toll-similar receptor signaling inhibits structural development of the distal fetal mouse lung. Dev Dyn (2005) 233(ii):553–61. doi:10.1002/dvdy.20362
PubMed Abstract | CrossRef Full Text | Google Scholar
142. Wan H, Kaestner KH, Ang South-L, Ikegami M, Finkelman FD, Stahlman MT, et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development (2004) 131(4):953–64. doi:10.1242/dev.00966
PubMed Abstract | CrossRef Total Text | Google Scholar
143. Jones CV, Williams TM, Walker KA, Dickinson H, Sakkal S, Rumballe BA, et al. M2 macrophage polarisation is associated with alveolar formation during postnatal lung evolution. Respir Res (2013) 14:41. doi:10.1186/1465-9921-14-41
PubMed Abstruse | CrossRef Full Text | Google Scholar
144. Tang X, Liu XJ, Tian C, Su Q, Lei Y, Wu Q, et al. Foxa2 regulates leukotrienes to inhibit Th2-mediated pulmonary inflammation. Am J Respir Cell Mol Biol (2013) 49(6):960–70. doi:x.1165/rcmb.2013-0122OC
PubMed Abstract | CrossRef Full Text | Google Scholar
145. Lambert L. Immunology of the Neonatal Lung and the Long Term Consequences of Neonatal Respiratory Virus Infection for Pulmonary Innate Immunity. Ph.D. thesis, Majestic Higher London (2015).
Google Scholar
campbelloffam1958.blogspot.com
Source: https://www.frontiersin.org/articles/10.3389/fimmu.2017.01570/full
0 Response to "Boosts Immune Response to Lung Infections and Promotes Easy Breathing"
Post a Comment